In the previous chapter you learnt about matter. The idea of divisibility of matter was considered long back in India around 500 B.C. Maharishi Kanad, an Indian Philosopher discussed it in his Darshan (Vaisesik Darshan). He said if we go on dividing matter, we shall get smaller and smaller particles. A stage would come beyond which further division will not be possible. He named these particles as ‘PARMANU’. This concept was further elaborated by another Indian philosopher, Pakudha Katyayan. Katyayan said that these particles normally exist in a combined form which gives us various forms of matter.
Around the same era, an ancient Greek philosopher Democritus (460 – 370 BC) and Leucippus suggested that if we go on dividing matter, a stage will come when further division of particles will not be possible. Democritus called these individual particles ‘atoms’ (which means indivisible). These ideas were based on philosophical considerations. Experimental work to validate these ideas could not be done till the eighteenth century. However, today we know what an atom is and how it is responsible for different properties of substances. In this chapter, we shall study about atoms and molecules and related aspects like atomic and molecular masses, mole concept and molar masses. We shall also learn how to write chemical formula of a compound.
OBJECTIVES
- After seen this video you will be able to :
- state the law of conservation of mass and law of constant proportions;
- list important features of Dalton’s atomic theory;
- distinguish between atoms and molecules;
- define isotopic mass, atomic mass, and molecular mass;
- define the mole concept and molar mass;
- represent some molecules with the help of a formula;
- apply the mole concept to chemical reaction and show a quantitative relationship between masses of reactants and products
- solve simple problems based on various concepts learnt.
Atom: The smallest particle of matter that cannot be further divided is called atom.
Molecules: Groups of two or more similar and dissimilar atoms that are linked by chemical bonds are called molecules. For example: O2, H2, N2, H2O, CO2 etc.
Element: The basic substance of a substance that cannot be simplified (broken) is called an element. Such as hydrogen, carbon, oxygen, iron and gold etc.
Compounds: Molecules that consist of more than one element are called compounds. For example: O2, H2, N2, H2O, CO2 etc
Click on download to read in Hindi
Concept of atom:
Indian philosopher:
- Maharishi Kanad:- According to them, when we divide the matter, small particles are obtained. Such fine particles will be obtained which will not be possible to divide again. He named these particles as Atoms.
- Pakudha Katyayan:- According to them, these particles are usually present in a combined form, which gives various forms of matter.
Greek philosopher
Democritus and Leucippus: According to them, if we go on dividing matter, a stage will come when further division of particles will not be possible. These individual particles are called atoms.
Laws of chemical combinations: This law is given by Antoine L. Lavoisier and Joseph L. Proust.
Law of conservation of mass : According to this law, in any reaction, the mass of the product and the mass of reactants used in it are equal.
Atoms and Molecules nios chapter 3rd (Part-1st)
(This rule was told by Antoine L. Lavoisier.)
OR
According to this law, mass can neither be created nor be destroyed it can only transfer from one form to another form in chemical reactions
Total mass of the reactants = Total mass of the product.
Part of chemical reaction
Reactant: The substance which take place in chemical reaction are called reactant.
Product: The new substance produced as a result of chemical are called products.

The law of constant proportions: Claude Berthollet and Joseph Proust worked on the mass of two elements. In 1808 Joseph Proust gave the law of definite or constant proportions.

According to this rule, in any compound certain types of elements are mixed in proportion to a certain mass. It does not depend on the method of its preparation or its source
Dalton’s atomic theory:
Dalton’s atomic theory, which was the concept of atom, called the smallest individual particle an atom. According to this law, “All matter, whether an element, a compound or a mixture, is made up of individual particles is called atoms”.
- All matter is made up of smallest indivisible particles.
- Atoms are smallest indivisible particles, which can neither be created nor destroyed in chemical reaction.
- All the given atoms have the same mass and chemical properties.
- Atoms of different elements have different mass and chemical properties
- Atoms of different elements combine to form a compound in the ratio of small whole numbers.
- The relative number and type of atoms in any compound are fixed.
The law of multiple proportions : According to this law “when two elements form more than one compounds, the masses of one element in these compounds for a fixed mass of the other element are in the ratio of small whole number.
For example, carbon and oxygen combine to form compounds. Carbon Monoxide and Carbon Dioxide etc.
Size of an atom: Atom size is very small, so small that if we keep millions of atoms, then it will be as thick as a layer of paper. Its size is (1 nanometer = 10-9 meters).
Atomic Mass: The relative atom of an element is the ratio of the average mass of its atoms to the mass 1/12th of the carbon-12 atom is called the atomic mass. The atomic mass is represented by the ‘u’ unified mass.
Atoms and Molecules nios chapter 3rd (Part-2nd)
Examples:
- Atomic mass of oxygen (O) = 16u
- Mass of sodium (Na) = 23u
Structure of an atom: Atoms are made up of individual fundamental particles electron, protron and neutron. The charge of the electron is opposite to the charge of the protron. Therefore, an atom is electrically neutral. The proton is present in the nucleus of an atom.
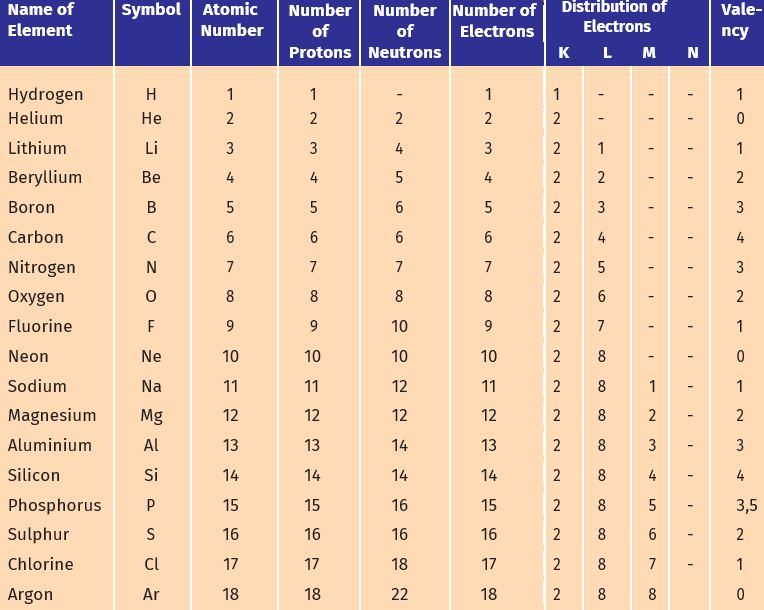
Atomic number (Z): The number of protons present in the nucleus of an atom is called atomic number.
(Atomic number = Electron number = Proton number)
Mass number (A): The sum of the total number of protons and neutrons present in the nucleus of an atom is called mass number.
(Mass number = protron number + neutron number)
Isotopes: Atoms whose atomic numbers are equal and mass numbers are different, it is called isotopes.

Average mass unit: isotopic of chlorine


Molecules: Groups of two or more similar and dissimilar atoms that are linked by chemical bonds are called molecules. For example: O2, H2, N2, H2O, CO2 etc.
Polyatomic Molecules: A molecule consisting of three or more atoms is called polyatomic. For example, phosphorus (P4), Sulphur (S8), carbon compounds (C60) (also called Buck minister fullerene), ethyl alcohol (C2H5OH).
Molecular Mass: The molecular mass of any molecule is the sum of the atomic masses of all the atoms present in that molecule. For example: molecular mass of carbon dioxide (CO2).
- Atoms present = 1 carbon atom + 2 oxygen atoms
- Total atoms present in the molecule = 3 atoms
- Mass of 1 carbon atom = 12g
- Mass of 1 oxygen atom = 16g
- Mass of 2 oxygen atom = 2 × 16 = 32g
Molecular mass (CO2) = mass of 1 carbon atom + mass of 2 oxygen atoms
Molecular mass (CO2) = 12g + 32g = 44g

Mole Concept:
Mole : The term mole was first used in 1896 by Wilhelm Ostwald. The word originates from the Latin word ‘moles’, which means ‘heap’. According to this, the calculation of molecules and atoms chemically is called mole.
Concept: A mole is the amount of substance that contains as many fundamental particles as there are atoms in carbon-12 isotopes.

Basic formula of mole

Molar mass: The mass of one mole of any substance is called its molar mass. For example, N2 is a mole of nitrogen of mass of 28 g/mol.
Molar mass is equal to molecular mass and atomic mass, but their S.I unit is gram (g).
Atoms and Molecules nios chapter 3rd (Part-3rd)
Writing chemical formula of compounds:

Formula of ionic compound:


Atomicity
The number of atoms present in a single molecule is called atomicity. For example, O2 is the atomicity of oxygen 2, similarly the atomicity of N2 is also 2.