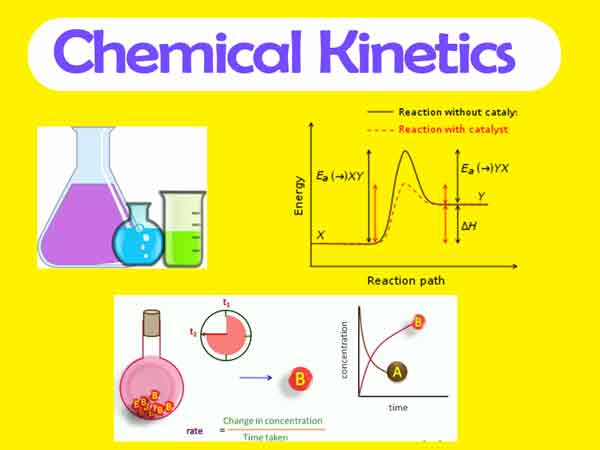
It is the branch of chemistry which deals with study of reaction rate and their mechanism.
Rate of Reaction:
R – P
H2 + l2 = 2HI

Factor affecting rate of reaction:
- Concentration of reactant
- Temperature
- Catalyst
➢ Dependence of rate on concentration:
Rate law expression: It is the expression in which reaction rate is given in term of molar concentrate of reactant with each term raised to some power which may or may not equal to stoic metric coefficient of the reacting species in a balanced chemical equation.



➢Integrated rate equation:
- Zero order reaction:



Half – life period:






